CCassured, fully named CRISPR Commons Assured, is a startup company stationed in the Netherlands in a small village named Ulvenhout, near Breda. CCassured is the frontrunner in CRISPR-Cas-related breakthroughs in diagnostics. CRISPR-Cas is an adaptive immune system in bacteria and archaea that destroys genetic material of foreign invaders in a precise manner. Scientists have repurposed this system and its precision for gene therapy and diagnostics applications. The owners of CCassured (Rogier Louwen and Sanne Voogd) are focusing with CRISPR-Cas on diagnostics, and using their CRISPR-Cas-based diagnostic platform and the ASSURED criteria standing for Affordable, Sensitive, Specific, User-friendly, Rapid, Equipment-free and Delivered, their developed tools can be performed at the point of care (POCT) and at low costs (less than 25 euro’s), e.g., for genetic colorectal cancer (CRC) screening.

CRC is one of the most common cancers worldwide and the second leading cause of cancer-related deaths. Early diagnosis is imperative to successfully implementing intervention strategies. Nowadays applied diagnostics are expensive, sometimes costing on average a 1000 euro per genetic CRC screening test, depending on the complexity and the number of genes being analyzed.
At-home screening tests are available, like FIT and Cologuard, that cost around 25 to 120 euro’s, respectively, but these tests still lack the required accuracy. Cologuard and FIT are fecal tests that measure the quantity of blood in stool, a possible sign of colorectal cancer. In addition, Cologuard also looks at DNA mutations associated with cancer found in cells shed by the lining of the colon. Overall, such non-invasive alternatives offer more comfort, and thus higher patient participation rates, but lack the sensitivity and specificity necessary for accurate detection. These limitations lead to false positives and negatives, risking unnecessary or missed follow-ups for early intervention, which are crucial for successful treatment of CRC. A significant number of patients also still end up unnecessarily in the hospital for more standardized procedures like colonoscopy – a procedure that is effective, but harbors significant risks, since its invasive nature leads to increased chances of bleedings and infections.
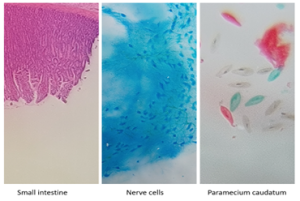
CCassured is dedicated to using its expertise and CRISPR-Cas-based platform to develop better performing POCT tests, with an increased sensitivity and specificity, while maintaining their affordability. Currently, CCassured can separate CRC tissue from healthy tissue with more than 99% sensitivity and specificity, and are further exploring with one of the ONOCSCREEN partners whether this can be applied to CRISPR-Cas-based technology for non-invasive methods by exploring the usage of liquid biopsies. For this purpose, CCassured has already successfully generated four POCTs (or ONCO-CRISPR prototypes) within the ONCOSCREEN project. These enable the detection of different CRC genetic biomarkers by using a simple dipstick method in plasma, in which free circulating tumor DNA is present. A cheap and easy-to-use home build (fluorescent) microscope has also been developed for less than 300 euro, pictures from which can be seen below. This microscope will support the CRISPR-Cas-based diagnostic detection methods in tissue. In the coming months the four ONCO-CRISPR prototypes will be further validated in an observational study, together with the ONCOSCREEN partners, reaching s key milestone within the ONCOSCREEN project!