Bringing Practical tools to patients
Welcome to the Oncoscreen Patient Portal
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Curabitur posuere sapien dolor, sed vestibulum augue consectetur sed. Morbi congue rutrum ipsum, fringilla convallis dui placerat nec. Ut in est odio. Ut efficitur ipsum at turpis sodales hendrerit. Integer sed sapien quis justo sollicitudin euismod vitae quis ante. Donec tristique viverra pellentesque. Nulla quis ornare sapien. Fusce volutpat dui dignissim tempor volutpat. Orci varius natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.
Praesent nisl lacus, mollis nec nunc ut, malesuada efficitur eros. Nunc eget vestibulum elit. Nullam ultricies eget orci ut aliquet. Nunc sed magna vitae eros dapibus finibus. Cras vel sollicitudin mi. Fusce lobortis, nisi vitae tempor interdum, est leo porttitor ante, a malesuada est ligula at nulla. Maecenas consequat malesuada ligula vitae luctus. Aenean sapien turpis, consequat eget odio eget, porta mattis est. Aenean eu lectus urna.
Pellentesque cursus dolor ac egestas elementum. In fermentum gravida neque et mattis. Praesent rhoncus leo id ligula eleifend, eu fringilla ex facilisis. Donec mi lacus, laoreet id enim a, porta cursus orci. Orci varius natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Praesent at nisi ut sapien consectetur mattis. Pellentesque vitae rhoncus elit, ac rutrum lectus. Mauris laoreet felis a vulputate venenatis. Mauris tempus, dui eget sollicitudin pharetra, turpis neque placerat elit, eget condimentum neque massa et ex. Maecenas quis massa a nunc lobortis facilisis. Fusce luctus in risus a varius. Nulla facilisi. Proin tempus vitae quam id bibendum. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. In volutpat venenatis tortor eget blandit. Praesent massa elit, lacinia vel finibus vel, tincidunt at urna.
Praesent pharetra turpis ut metus molestie consequat. In quis aliquet ante, ut aliquet enim. Maecenas interdum nisl non leo placerat, luctus scelerisque velit venenatis. Duis vestibulum justo porta justo pellentesque, ac accumsan diam varius. Ut urna orci, lobortis quis eros sed, accumsan blandit nulla. Integer at arcu euismod, ullamcorper arcu ac, molestie tortor. Aliquam erat volutpat. Nulla iaculis orci neque, vel consequat metus laoreet sed. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut nec porttitor velit, ut elementum tellus.
Quisque dictum enim in quam venenatis, vitae fringilla massa hendrerit. Donec at dapibus elit. Integer id mauris risus. Phasellus lobortis lorem sit amet sem facilisis, et cursus dolor lobortis. Curabitur vehicula placerat enim, vitae cursus arcu mattis ut. Morbi ullamcorper erat sed mauris dapibus dapibus. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Donec sollicitudin sem vel elit convallis blandit. Proin maximus, eros eget condimentum porta, turpis nunc dapibus nisl, eu ornare mi ipsum nec lacus. Maecenas non lectus quis elit placerat dapibus sit amet vitae purus. Nam orci dolor, vulputate a placerat sed, pulvinar at tellus. Praesent nec sapien luctus, faucibus est quis, tincidunt dolor. Pellentesque eu lorem nec tellus blandit varius. Etiam efficitur finibus nisl in molestie.
CLINICAL TRIALS
ONCO CTC: CTCs as epithelial cancer cells are able to move, migrate and invade blood vessels after epithelialmesenchymal transition (EMT) and have been characterized as the main causal factor of tumor metastasis mediation. Compared to other cancer biomarkers, CTCs contain molecular and biological information about the tumor as a whole, supporting single cell analysis and ongoing changes in tumors at all stages. CTCs can be found in blood as independent cells, and clusters of both CTCs alone and cells aggregates comprising of neutrophils, platelets, and CTCs. Thus, blood-based analysis of CTCs could therefore function as a “liquid biopsy,” allowing repeated sampling.
ONCO NMR: The method has been utilized extensively in other cancer settings, in particular for breast cancer, and recently also for colorectal cancer where the specificity and sensitivity were between 82 to 85%. (Vignoli et al., 2021). UzL has developed a standardized NMR test for the analysis of blood serum and plasma samples.

ONCO CRISPER: The ONCO-CRISP is a screening test based on CRISPR-Cas9 biomarkers. The ONCO-CRISPR tool and human CRISPR microRNAs will enable the detection of clinically relevant biomarkers in colorectal adenomas, colorectal carcinomas in tissue and non- or minimal invasive obtained patient samples. The test consists of a detection dipstick, a reporter, a Cas protein (nuclease, DNA/RNA cutting enzyme) and a guiding RNA, input material that could be RNA or DNA that contains the biomarker of interest, and more. The test will show a negative or a positive result on a used test strip. Testing will be done according to the testing instructions.

ONCO VOC: The ONCO-VOC is a screening test based on Volatile Organic Compounds (VOC). ONCO VOC, The innovative breath analyzer developed by TECHNION, is based on an 8-sensor array of nanomaterials in conjugation with AI. To perform the test a short-exhaled breath sample (2-3sec.) will be evaluated in less than 2 minutes. The results of this test will give an early indication for distinguishing between CRC-linked and healthy categories.
Patient Portal Map
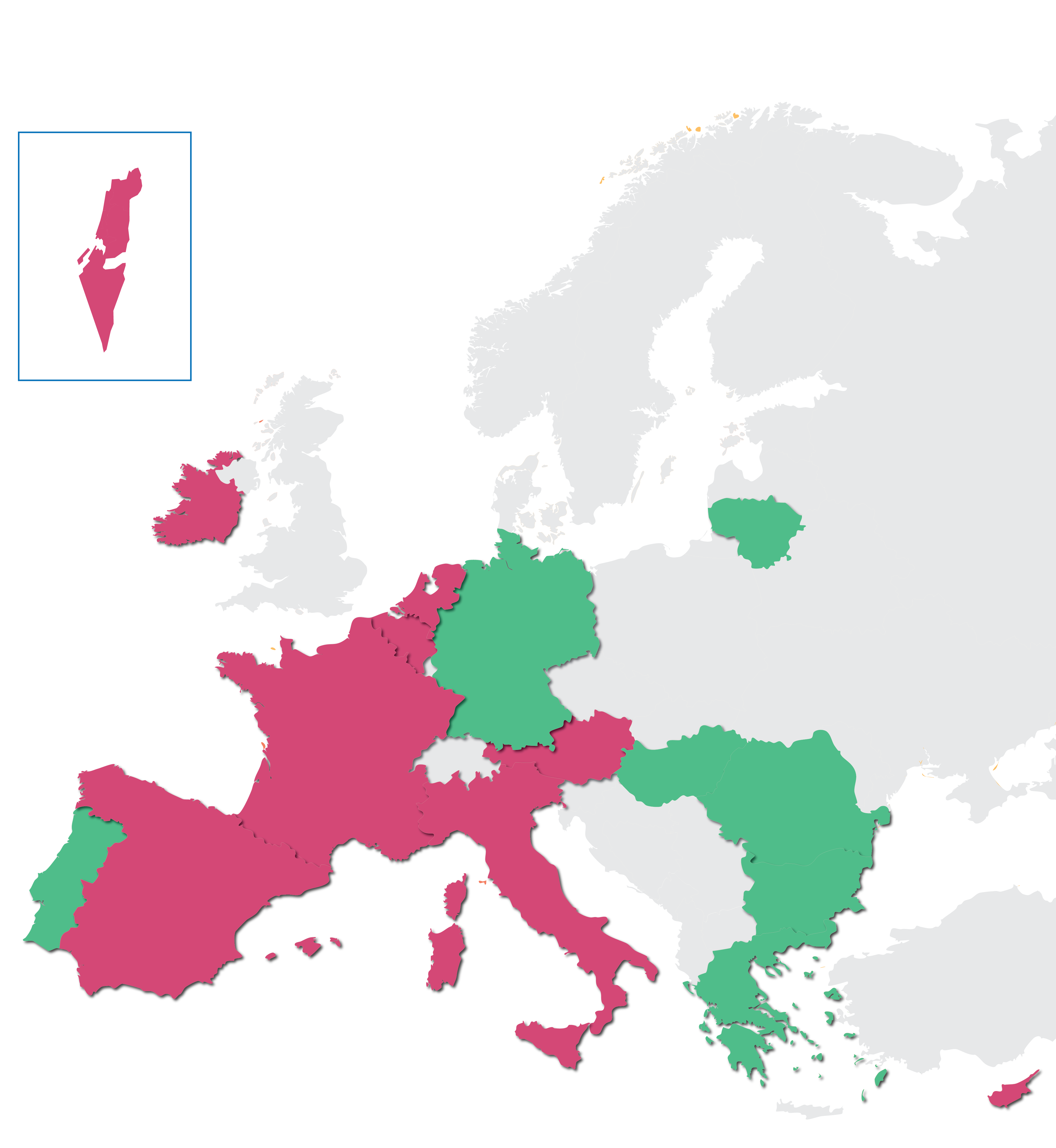
PORTUGAL
BULGARIA
University Specialized Hospital for Active Treatment of Oncology
Contact Person: Dr. Bojidar Tomov
Clinical Trials: ONCO VOC, ONCO NMR, ONCO CRISPR
-------------------------------
Military Medical Academy Sofia
Contact Person: Dr. Daniela Stoyanova
Clinical Trials: ONCO VOC, ONCO NMR, ONCO CRISPR
LITHUANIA
GERMANY
UMC MAINZ
Contact Persons: Markus Moehler ; Katja Petrowski
Clinical Trials: ONCO VOC, NMR, and CRISPR
------------------------------
Lübeck UKSH
Contact Person: Birgitta Spitzner
Clinical Trials: ONCO VOC, NMR, and CRISPR
HUNGARY
University of Szeged
Contact Person: Prof. Dr. Tamás Molnár DSc
Clinical Trials: ONCO NMR and CRISPR
----------------------------
Semmelweis University
Contact Person: Dr. ILIÁS Ákos, PhD
Clinical Trials: ONCO NMR and CRISPR
GREECE
AHEPA University Hospital
Contact Person: Dr. Antonis Billis
Clinical Trials: ONCO VOC, NMR, and CRISPR
ROMANIA
CONTACT YOUR NEAREST TRIAL SITE
Rosenbaum Group
Want to learn More?
Newsletter Signup
To keep up-to-date with the latest developments in the ONCOSCREEN project, subscribe to our LinkedIn newsletter.
You can always unsubscribe at a later time if you change your mind.




.png)